Sisonke Vaccine Registration Online : sisonkestudy.samrc.ac.za
Organization Name : Department of Health
Facility Name : Sisonke Vaccine Registration Online
Applicable For : Health Care Workers
Website : http://sisonkestudy.samrc.ac.za/
| Want to comment on this post? Go to bottom of this page. |
|---|
Sisonke Vaccine Registration Online
The Sisonke Programme is a collaboration between the National Department of Health, South African Medical Research Council, Desmond Tutu Health Foundation, CAPRISA, Janssen and Johnson & Johnson.
Related / Similar Service : Sisonke Vaccine FAQs

It allows the government to make the Ad26.COV2.S COVID-19 vaccine (JnJ vaccine) immediately available to healthcare workers using a research programme.
Sisonke is not the same as a clinical trial. Rather it is a way that research can help to make a vaccine available while the licensing process takes place.
A third wave of COVID-19 is predicted to begin in South Africa this winter. Protecting healthcare workers is a priority and so we must start vaccinating our healthcare workers before the third wave arrives.
Who Can Take Part?
To be vaccinated in a hospital you need to be :
** Age 18 and older AND
** A health care worker in the private or public service AND
** Willing and able to comply vaccination plan and other study procedures. AND
** Capable of giving electronic or personal signed informed consent as described in Appendix 5, which includes compliance with the requirements in this protocol.
To be included in a detailed sub-cohort you need to be :
** Age 18 and older AND
** Health care worker in the private or public service AND
** Willingness and ability to comply with all scheduled visits, vaccination plan, laboratory tests, and other study procedures, with follow-up at an ENSEMBLE site. AND
** Capable of giving electronic or personal signed informed consent as described in Appendix 5, which includes compliance with the requirements in this protocol.
You cannot receive the vaccine if you :
** Have any significant acute or chronic medical condition, situation or circumstance that in the opinion of the PI/designee makes the participant unsuitable for participation in the study, or jeopardises the safety or rights of the participant
** Are or may be pregnant at time of enrolment or planning within 3 months.
** Currently participate in any other research studies that would interfere with the objectives of this study.
The determination of whether participation in another study would be exclusionary for a given participant will be made by the PI/designee
** Have a history of severe adverse reaction associated with a vaccine and/or severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
How It Works?
** The South African government has secured half a million doses of the vaccine from JnJ, enough to vaccinate half a million health workers.
** The first batch of 80,000 doses arrived on the 16th of February and further deliveries will follow every two weeks.
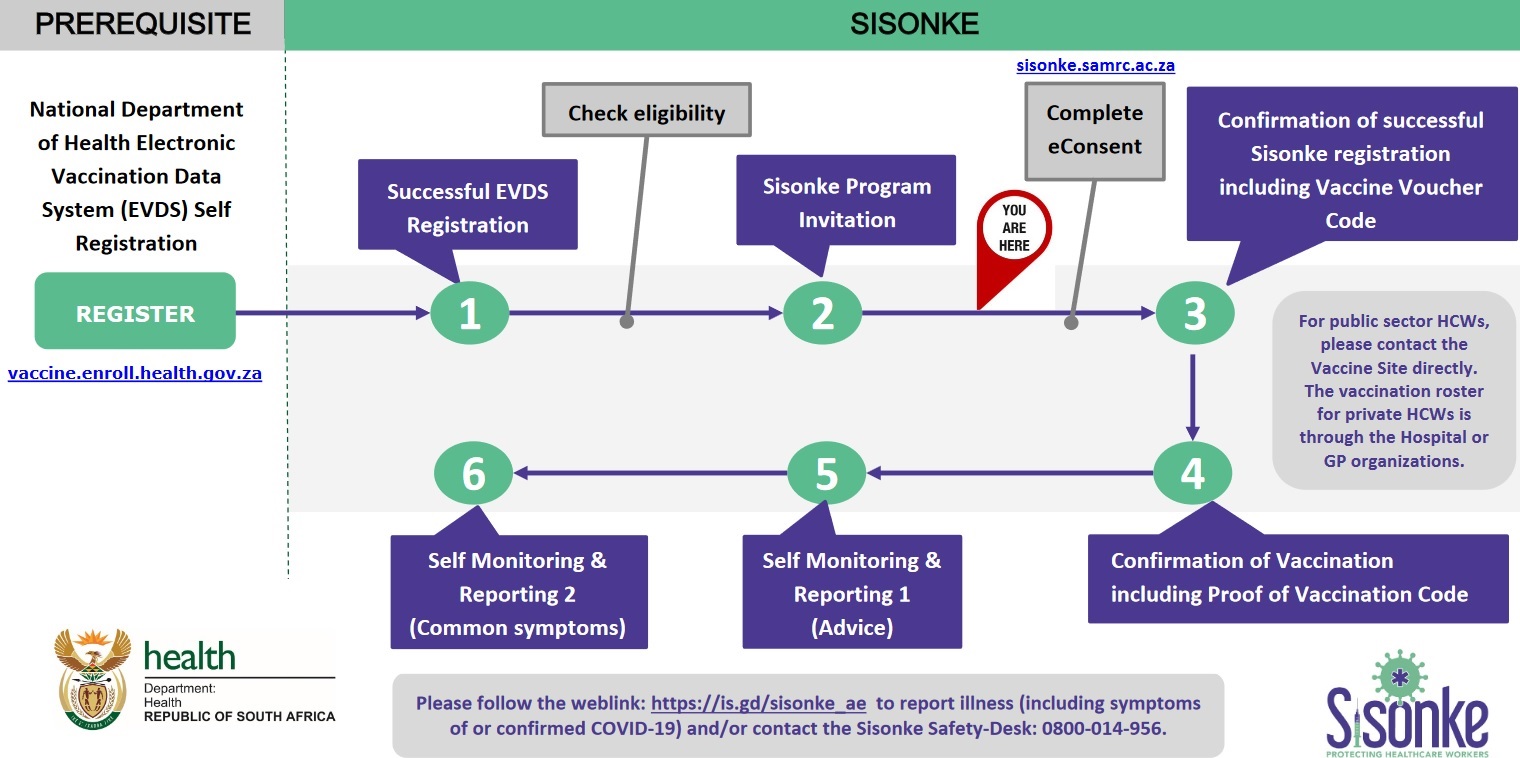
** Vaccines will be available at 17 hospitals throughout the country where teams of researchers and vaccinators will work together to deliver the vaccine to health workers up to 10 hours a day, 7 days a week.
** Research staff will be responsible for ensuring the cold chain and drawing up the correct amount of vaccine. Vaccinators will complete assessment checks, administer the vaccine and monitor you for a few minutes.
** The first step to accessing a vaccine is to register on the country’s Electronic Vaccination Data System (EVDS). You can access the system by visiting this website: https://vaccine.enroll.health.gov.za
Contact Us
Sisonke desk – 0800 014 956
FAQ On Sisonke Vaccine Registration
Here are some FAQs related to the Sisonke Vaccine Registration process:
Q: Is the Sisonke Vaccine study safe?
A: The Sisonke Vaccine study is a clinical trial that has been approved by the South African Health Products Regulatory Authority (SAHPRA) and an independent ethics committee. The study vaccine has been tested in clinical trials and was found to be safe and effective in preventing COVID-19.
Q: What type of vaccine is used in the Sisonke study?
A: The Sisonke study uses the Johnson & Johnson COVID-19 vaccine.
Q: Is the Sisonke Vaccine study mandatory?
A: Participation in the Sisonke study was voluntary. Healthcare workers who met the eligibility criteria were given the option to participate.
Q: How many doses of the vaccine are given in the Sisonke study?
A: The Johnson & Johnson COVID-19 vaccine used in the Sisonke study is a single-dose vaccine.
I have not received sms msg to register for reboorst,still waiting for msg
I also registered but idid get voucher number please help
I am 49 years old (ID 7202280740080) and was diagnosed with Pulmonary Embolism Blood Clot in my right lung in January and currently on Warfarin so is it safe for me to be vaccinated?
And can I register and be vaccinated with the elderly or can I wait and be vaccinated within any other category.
My Cell Number 27607895122 I have been registered since March, on EVDS I SMS that says I was successfully registered My ID 6302145053080 still have not received my voucher number and I am a frontline worker. Where do I go from here?
I REGISTERED WITH TWO DIEFFERENT CELL PHONE NUMBERS THE FIRST ONE IS NOT WORKING I DIDI NOT RECEIVE ANY SMS
MY ID NO 6808090620089
WORKING AT NICD
I received sms that I have been registered but no Voucher number was given need to know how to go about to obtain the Voucher number.
Alma Olivier – id number 5906260110088 – working for Netcare Hospitals central offices Sunninghill.